Distinguish Distillation & Extraction with application.
Distinguish Distillation & Extraction with application.
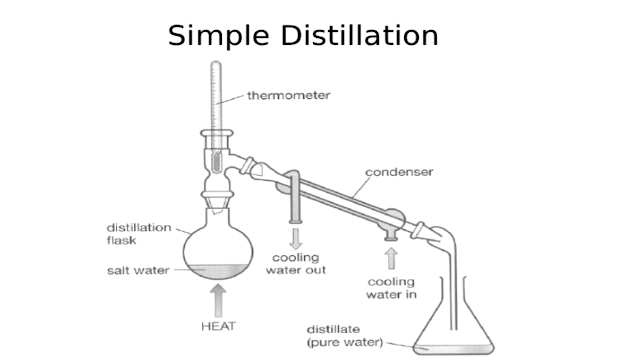
Distillation
Distillation is a widely used method for separating mixtures based on differences in the conditions required to change the phase of components of the mixture. To separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase. The gas is then condensed back into liquid form and collected. Repeating the process on the collected liquid to improve the purity of the product is called double distillation. Although the term is most commonly applied to liquids, the reverse process can be used to separate gases by liquefying components using changes in temperature and/or pressure.
A plant that performs distillation is called a distillery. The apparatus used to perform distillation is called a still.
Uses of Distillation
Distillation is used for many commercial processes, such as the production of gasoline, distilled water, xylene, alcohol, paraffin, kerosene, and many other liquids. Gas may be liquefied and separate. For example: nitrogen, oxygen, and argon are distilled from air.
Applications
- Sepration of volatile oil
- Purification of organic solvent
- Refining of petroleum products
- Seperation of drug obtained from plant and animal.
- Purification of drug from animal source.
Comments
Post a Comment